Water/H2O is the most abandoned single compound so the properties of water are most discussed in the universe. It is also essential for all living matters. mineralogical evidence has shown that liquid water must have existed 4.404 ± 0.008 billion years ago, so Just after the formation of Earth
Till 1871 water was considered an element. But Cavendish proved that it is not an element but a compound. Also, he exploded inflammable air and produce a drop of water H2O. It is a compound of oxygen and hydrogen. It is a universal solvent. Also, It is found in three states
Water/H2O
Ice↔water↔steam
Basically, 75% of the universe is consistent with H2O. Also, the human body contains 70% of water. Equally, vegetables and fruits contain up to 80% of water. The watermelon e.g. contains 99% H2O. All universes contain about 75% of water. Water is a universal solvent so it is not found pure in nature.
Another key point, it is used for drinking and washing in everyday life. It is largely used in industries.
Physical properties of water
- It is clear transparent and colourless, But if we watch 2-meter depth, it is bluish-green.
- Pure water has no odour and is also tasteless. It has some taste due to the presence of some salt.
- Its freezing point is 00C, and also it melts at this temp. I.e water to Ice and vice versa, at 00C and 1 atmospheric pressure(atm).
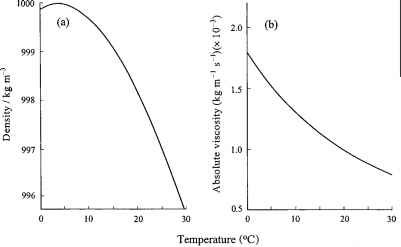
Water density and viscosity The boiling point of water
- It has a high boiling point of 1000C at 1 atm pressure and also on increasing pressure, It decreases by 5 parts per million ml.
Density of water
5.Water has density 0.9168 gm/ml at 00C, which has max value 1 gm/ml at 3.98oC.
6. From 00C to 3.98oC it contracts and above or below it expands. Expand← 00C→ 3.98oC→expand
7. Since water expands on cooling and may be causing the bursting of pipes in winter. Water can be cooled up to 200C called supercooled pressure.
latent heat of fusion of water
8. The latent heatof fusion (Hf)of ice is 79.74 cal/gm.
9. It has a dipole moment of 1.80 debye
10. Abnormal behaviour of water/H2O behaves abnormally from other hydrides
| Hydrides | B.P0C | M.P 0C | Hf | Kcal/mol, H Evap |
| H2O | 100 | 0 | 1.44 | 9.72 |
| H2S | -60.3 | -85.5 | 5.7 | 4.46 |
| H2Se | -41.3 | -65.7 | 0.6 | 4.62 |
| H2Te | -2.2 | -51 | 1.67 | 5.55 |
It has dipole movement at normal temperature because oxygen is more electronegative than other members.
Hydrogen bonding of water
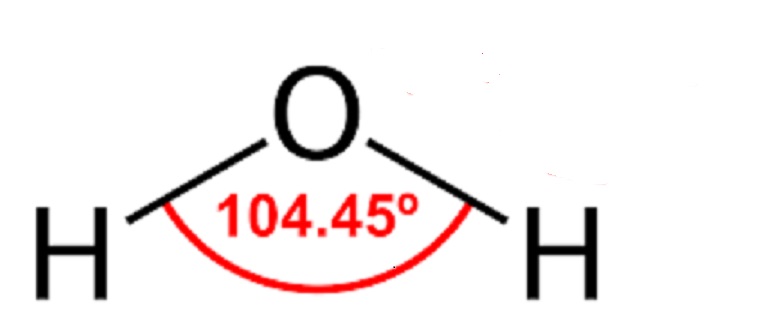
11. The range between two hydrogens in water is 104.50 and also hydrogen bonding has energy(O—H) of 40 Kcal/mol.
Dielectric constant and surface tension of water
12. It behaves abnormally; it has a dielectric constant of 80 and surface tension of 72 dynes/cm2 other than the liquid of its family
Universal solvent
13. Water is a universal solvent it dissolves at low temperatures as well as high temperatures. Some compounds when dissolved evolves heat i.e. exothermic reaction due to solvolysis.
H2SO4 + H2O
H2SO4 forms are monohydric as well as bi-hydrous. All molecules of H2SO4, when dissolved in water, evolve heat and the water turn to steam so the splash out of the pot becomes, When NH4Cl is dissolved in water reaction is endothermic i-e it gets heat from the water and causes cooling hence temperature decreases.
14. When an acid is added it ionizes
H2O ↔ H++ H–
It is quite stable but when exposed to ultraviolet light it decomposes
H2O↔ H2+ 1/2O2
H2O+ 1/2O2↔ H2O2
15. Water has a viscosity of 0.018 at 00C
16. So, some of the salts from hydrates with H2O
AlCl3.6H2O ,CuSO4.5H2O , Na2Co3.10H2O
This can be eliminated by heating
17. H2O in liquid form may be assumed to contain associate molecules (H2O)2 and (H2O)3. which is shown by the Raman effect and also other physical observations.
Chemical properties of water
- Firstly, all the water has a ratio of two gases hydrogen and oxygen 2:1 by volume and 1:8 by weight

i.e H2O
(2:1) volume
(1:8) weight
2. So, It is neutral having a PH value of 7. Also, Pure water H2O exists as an equilibrium between the acid and alkalies as PH. All in all, In pure-form water, the acid concentration equals the hydroxyl concentration and both are present at 7-10 gram equivalents (or moles) per liter.
3. Reaction with 1st group metals Na, K Cs, Rb, and also with other high electropositive metals Ca, Sr, Ba, etc. is vigorous. So, these react in the cold state with H2O
2Na + 2H2O → 2NaOH + H2↑
2K + 2H2O → 2KOH + H2↑
2Ca + 2H2O → Ca(OH)2 + H2↑
Also, i.e. alkalis are formed and hydrogen is evolved.
Formation of hydroxides or oxides
4. Also properties of water its low electropositive metals Mg, Fe, Zn, and Cu, which react with water in hot form and form hydroxides or oxides.
Mg + H2O → MgO + H2↑
Mg + 2H2O → Mg(OH)2 + H2↑
Fe + H2O → FeO + H2↑
FeO + H2O → Fe(OH)2↑
Zn + 2H2O → Zn(OH)2 + H2↑
Cu + 2H2O → Cu(OH)2 + H2↑
5. Also, some of the metals do not react with water e.g. Au, Ag, Sn
Metal oxides reacting with H2O
6. All of a sudden, metal oxides reacting with H2O form alkalis and non-metal oxides form acids. it is very important in the properties of water
Na2O + H2O → 2NaOH
CaO + H2O → Ca(OH)2
SO2 + H2O → H2SO3( sulphurous acid)
SO3 + H2O → H2SO4( sulphuric acid)
CO2 + H2O → H2CO3
P2O5 + 3H2O→ 2H3PO4 (orthophosphoric acid)
P2O5 + H2O→ 2HPO3 (metaphosphoric acid)
N2O5 + H2O→ 2HNO3
Non-Metal reacting with H2O
7. Hence, Non-metal reacts with H2O at high temperature
C (coal) + H2O→ CO + H2 (water gas)
As a result, water gas is powerful fuel
8. Gases also react with water (H2O) when dissolved give
Cl2 + H2O → HCl + HOCl
2HOCl + O2 → 2HCl + 2O2
F2 + H2O → H2F2 + 1/2O2
NH3 + H2O → NH4OH
Water decomposes in the presence of ultraviolet rays
9. Also, water decomposes at high temperatures and in the presence of ultraviolet rays.
H2O ↔ H2+ 1/2O2
At 20000C and 1 atm only 0.6% but at 35000C about 30%
Also, in the case of U.V rays too
H2O ↔ H2+ 1/2O2
H2O + 1/2O2↔ H2O2
Ionization of water
10. Although in the presence of acid, it ionizes
H2O ↔ H++ H–
11. With CH4 also water gas is obtained
CH4 + H2O→ CO + 3H2 (water gas) in the presence of Ni at 9000C
12. Also, some salts form hydrates with water. AlCl3.6H2O, CuSO4.5H2O, FeSO4.7H2O etc. so can be eliminated by heating.
Efflorescence
Some hydrated salts (substances) have vapour pressure greater than atmospheric pressure hence water molecules escape into the air. All in all these salts are called efflorescent and the phenomenon is called efflorescence.
Deliquescence
Some salts have no water molecules (anhydrous) but their vapour pressure is less than atmospheric pressure, they get water molecules these salts are called deliquescent and the phenomenon is called deliquescence.