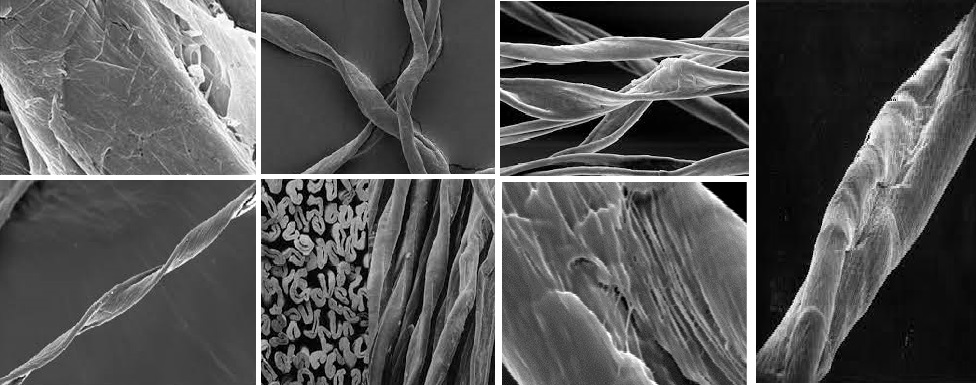
What are cotton’s properties?
The properties of cotton are the most important in fiber. Moreover, It is the backbone of all textile industry trade. Basically, cotton fiber is obtained from seed cotton. Cotton is mainly composed of cellulose. It belongs to the “Gysspium” family. Also, it is cultivated in tropical areas like Pakistan, India, Egypt, and the U.S.A.
Physical properties of cotton
Below are described the physical properties of cotton.
Color of cotton
The color of cotton is normally creamy white. If the picking of cotton is delayed then it may be turned to grey, yellow, or bullish white.
Strength of cotton
Cotton fiber is a moderately strong, structure of cotton fiber due to the intrinsic structure of layers of cress-crossed minutes-spirited fiber that compose the fiber cell. Also, the strength of cotton is determined by the character of the cotton yarn which should be a long staple and tightly twisted. Moreover, it is affected by moisture content. Hence, the strength of the cotton fiber is expressed in tensile strength and tenacity
Cotton has a tenacity of 3~5 grams per denier. Generally, it has a tensile strength of 40,000 to 120,000 pounds per inch. Also, the strength of cotton is very important and can be improved by Mercerization. Generally, cotton strength is checked by a “Pressley strength tester”.
Tensile Strength of Cotton
Cotton quality depends on its tensile strength e.g
| Tensile strength ( 103 ponds per inches2) | Grading |
| 89~97 | Strong |
| 81~88 | Average |
| 72~80 | Fair |
| 71~blow | Weak |
Elasticity of cotton
Cotton fiber has very little natural elasticity. Basically, this characteristic can be altered to varying extents by hard twisting the fibers into creped yarn and by using such fabric techniques as knitting. Also, at a 2% extension, it has an elastic recovery of 74%, and at a 5% extension, it has an elastic recovery of 47%.
Elongation at break of cotton fiber
When fiber is subjected to force this will stretch to some degree before it breaks, this stretching is called elongation at break. Cotton does not stretch easily. Generally, it has an elongation at a break of 5~10% depending upon a variety of cotton.
Specific gravity of cotton
Specific gravity is the ratio of mass measured in the air to the mass of an equal volume of water at 4oC, Also, the specific gravity of cotton is 1.54.
Fiber length of cotton
Staple length is the term used for the length of cotton. So, staple length is defined as the average length of the fiber in a mass of cotton. Also, on the basis of staple length cotton is classified into three classes.
S.L (1″~2 1/2″)
This type of length consists of fine twisterious fiber, top-quality sea island, and Egyptian cotton included in this class.
S.L (1/2″~1 5/16″)
This type of cotton is not as fine as, the American upland cotton of this class.
S.L (3/8″~1 “)
This type of cotton is of poor quality course fiber. Basically, it is produced largely in China, India, Pakistan, and to a smaller extent in Iran, Iraq, Turkey, etc.
Maturity of cotton fiber
The maturity of cotton is the degree to of cell walls of cotton fibers are developed. However, fiber maturity is not an estimate of the age of cotton plants. so, mature fiber will be well-developed cross-sections and be lustrous. Well-mature cotton fiber is mostly required in knitting and secondly for weaving.
The fineness of cotton fiber
It is defined as the weight of 1″ of fiber in grams. Also, the instrument used for checking the fineness of fiber is called a fibrograph.
| Fineness microgram per inch | Grading |
| Below 3 | Very fine |
| 3~3.9 | Fine |
| 4~4.9 | Average |
| 5~5.9 | Coarse |
| 6~above | Very coarse |
Chemical properties of cotton
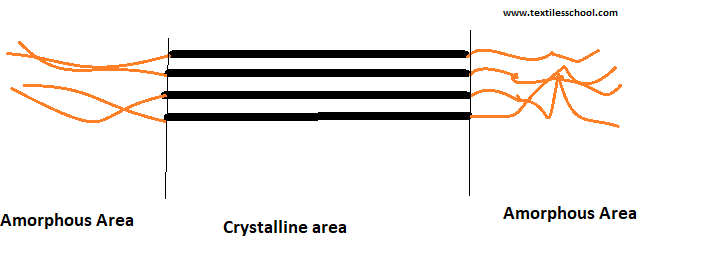
Cotton chemistry can be altered by using chemical treatments or finishes. Mercerization, for instance, is the process of treating yarn cotton or fabrics with sodium hydroxide for increasing luster, absorbency, softness, strength, and softness. Hence, in mercerization, the fiber wall swells and its cross-section becomes rounder. Also, the strength of the fibers increases remarkably, and in effect, extensibility decreases due to the swelling effect, which moreover, causes creep of the fiber and untwisting. Hence further reducing its degree of crystallinity.
Below are the chemical properties of cotton fibers.
Effect of acids
Cotton is not affected by cold dilute acids but hot dilute acids or concentrated acids disintegrate it. A 2-gram per liter of solution of H2 SO4 at 80 0C reduces the strength of fabric by 25% in one hour. It is, therefore, such acid should be used with low concentration and low temperature. The hydrolytic action of acids depends in large measure on temperature e.g a slight increase in temperature in processing (80~90oC) produces a stronger effect than when the concentration of H2 SO4 doubled
Effect of alkalies

Cotton has excellent resistance to alkalies, It Sewell into acoustic alkalies but is not damaged. Also, It can be washed separately in soap solution without harm. It is characteristic of the glucosidic linkage of the cellulose molecule that it is highly resistant to alkalies. At normal temperature, a weak solution of caustic soda lime has no effect on cellulose, but on boiling in NaOH a small part of cellulose passes into the solution, As the alkalies can increase the cellulose solubility, become considerably high.
The action of oxidizing and reduction agents
Reducing agents have no effect but oxidizing agents readily convert it into oxy cellulose. Also, for chemical treating fibrous material wide use of oxidizing agents. This regent causes less or more intensive oxidation of cellulose functional groups and breakage of chains as a result of glucoside linkage repature. The mixture of the different products of cellulose oxidation is usually called oxy-cellulose. The oxidizing regents are NaOCl, H2O2, NaCl, HNO3, etc.
The action of salts solution
The solution of acid salts has some effect on cellulose as acids. A similar effect is produced such as natural salts NaCl and MgCl2 which undergoes hydrolysis when treated in heating. Cellulose swells into some concentrated solution of some natural salts and on being gradually heated first becomes jelly and then passes into solutions. the solution of the following salts LiI, Li(NS), KCN, and Ca(CNS)2 strongly affect the swelling and resolving of cellulose. The ammoniacal solution of copper oxide is a specific solvent for cellulose.
Effect of organic solvent
There are few solvents that resolve cotton completely. So, it has high resistance to solutions like alcohol, ether, benzene, and gasoline. But is distressed by copper complexes, cuprammonium hydroxide, and cupric-ethylene diamine and by the concentration of 70% of H2 SO4.
Action of water
The dry cotton fiber constructed from its fiber of cellulose is fairly stiff and rigid ability. The cellulose molecules are held tightly together inside the fibrils bundle by a band established between molecules laying close side one another.
Also, water molecules tend to force the molecules of cellulose apart lessening the forces that held the cellulose molecules close together and destroying some of the rigidity of the entire cellulose structure. So, the glucoside linkage of cellulose molecules is readily hydrolyzed i-e they break up by combining with water which results in the disintegration of molecules
( C6 H10O5)n+n H2O to n(C6H12O6)
ware acts as a plasticizer for cotton
Effect of microorganisms
Cotton is attacked by fungi mildew and bacteria. Mildews are particularly troublesome on cotton that has been treated with starchy finishes. Mildew and bacteria will flourish on cotton, especially under hot moisture conditions.
Effect of heat
Cotton has excellent resistance to degradation by heat. So, It begins to turn yellow after several hours at 120oC and decomposes remarkably at 150oC. Cotton is severely damaged after a few minutes of 240oC.
Effect of sunlight
There is a gradual loss of strength when cotton is exposed to sunlight it turns yellow for a long time. So much damage is caused by ultraviolet light and by the shorter weave of the visible light
Uses of cotton fiber
Cotton fabrics are inexpensive and are largely used. However, the use of cotton may be classified into three main classes
Apparel uses
Trousers, shirts, pants, underwear, hosiery, etc.
Household uses
Bed sheets towels, curtains, rags, and carpet.
Industrial uses
Industrial threads bags, shoes, ropes, conveyors belts, medical supplies, and equipment