Sources of impurities and the use of water are important because it is found abundantly in the universe. Shortly pure sources of water are colourless and odourless, Then after that, impurities are added when running on the surface of the earth. There are three main natural sources of water.
1-Sky source
Firstly rainfall and also snowfall
2-Surface source or soil water
River, canals, streams, lakes, and sea or ocean water
3-Sub soil source
Tubewell, wells, and also springs
Sky source
At first, when water is obtained from sky sources in the form of rainfall or snowfall, it absorbs the roots particles in the air, dust, and many gases like O2, CO2, SO2, NH3, H2S, and oxides of N2due to lightning and bacteria. But this water is still the purest source of water.
Rainfall and snowfall can not be used for industrial purposes but they may be used for irrigation and many other purposes. It is not used in industry because it is inconsistent and in smaller amounts.
Surface source or soil water
Surface sources of water are in the type of rivers, canals, streams, lakes and sea or ocean water.
The rainfall water has impurities when falls on the earth’s surface so, it finds its way into rivers, streams, canals and lakes and to the ocean it gets impurities.
Subsoil water
In this source, we get water from wells, tube-well and springs.
Wells and tube-wells
During the rainy season, water travels on the surface of the earth and sinks down through the permeable stratum of the earth and gets stored or impermeable stratum.
This water is obtained by digging wells, tube-wells and the water obtained is fresh and has no suspended impurities but many dissolved impurities and mineral salts. It is odorless, bacteria free and drinkable. This water is industrially exploited. It also contains CO2 abundantly and is dependent on area.
Permeable stratum/ impermeable stratum
This means absorption of ration water into the soil due to gravity is a permeable stratum and reversely that soil which interferes with absorption is called an impermeable stratum, both reactions naturally occur and maintain ground and underground water
Spring water
Inside the earth where the permeable stratum is thin and gets closer to the impermeable stratum also, water rushes out from pores etc. This water is called spring water. It is usually found in mountain areas. There is no suspended impurity in it and also bacteria free but there are many mineral salts in it.
NaCl (saline), NaHCO3(alkalies), Mg SO4(bitter), CO2(created)
Spring H2O having CO2 are good for digestion. Spring H2O sulphur is sulphurated so well for skin disease.
Water containing Fe(HCO3) is called tonic and people in that area are usually healthy. This spring water is also exploited by human beings and industry.
“Water as the universal solvent”
Firstly described, Water H2O in pure form is colourless, tasteless, and odourless. its molecule is composed of hydrogen and oxygen. As a result, Water becomes contaminated by the substances when comes into contact. it is not available on earth for use in its pure state. Also, water can dissolve every natural substance on Earth. Due to this property, water has been termed a “universal solvent”. By all means, due to this property, water is a major threat to industrial equipment which includes corrosion reactions which are caused due to chemical reactions with metals, which produce scale on heat exchanger surfaces. Additionally to control the corrosion factor water requires purification up to the required level.
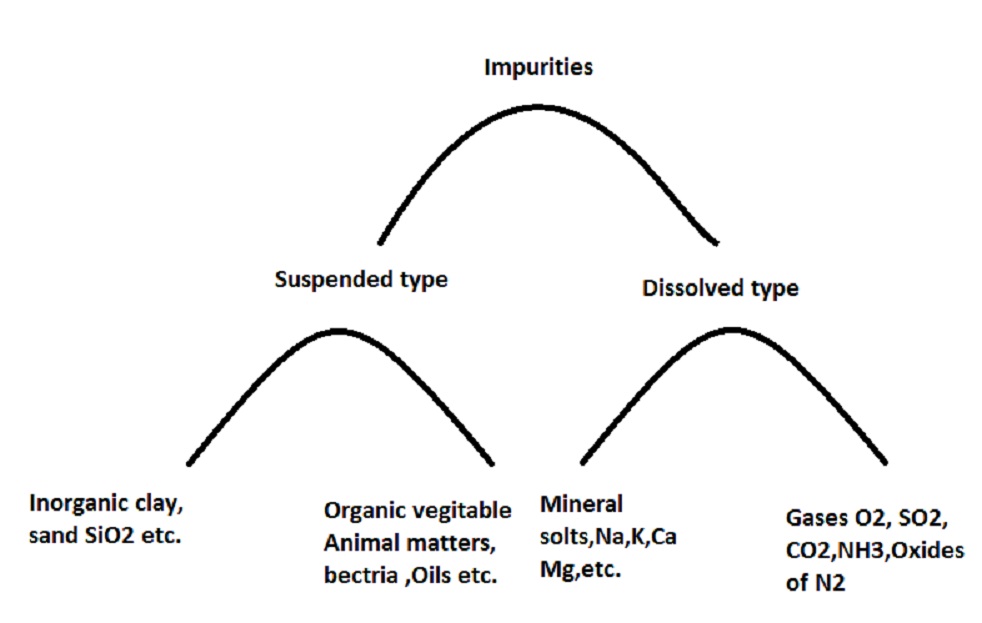
Impurities present in water
Impurities present in water mainly depend upon sources of water. Water is a universal solvent so pure water is rarely found. So, water impurities include dissolved and suspended solids. Also, Ca(HCO3)2 is a soluble salt called bicarbonate. Bicarbonate water is clear in appearance as it is present in atomic sizes and cannot reflect light, correspondingly, some can reflect this light and seems as cloudy. These particles usually impart visible turbidity to the water. Also Dissolved and suspended solids impurities are present in most. Sweetwater is somewhat cloudy due to sodium chloride salts being present
At this point, surface waters can be categorised as under.
- Physical/suspended impurities
- Bacteria/biological impurities.
- Chemical impurities, mineral salts/dissolved gases
- Inorganic impurities
As it passes on the surface of the earth it gets organic matter, certain amounts of mineral matter, salts and also many other suspended impurities.
Type of impurities
This water has two kinds of impurities.
Sea water is more impure than rivers canals, and streams, which take water and contain 3.6% of total dissolved solids. It also contains an excess of NaCl.
Soil water or surface source water is the dirtiest water and is used for sea animals and vegetables due to excess minerals. Also, this water is exploited industrially and many industries are set up near the river banks, canals and many lakes.
a) Physical/Suspended impurities
In physical impurities of water, we can summarise as colour, odour, taste and turbidity. Colour, odour and taste in water are due to the presence of organic matter, and minerals. Also, clay, vegetables, animal matter and other soil parts are present in water all parts are called turbidity.
b) Biological impurities
In suspended form (bacteria, algae, also fungus)
c) Chemical impurities
Dissolved gases such as O2, N2, CO2, SO2, CH4, H2S, etc
Also salt, oil, and metal oxides dissolved (HCO3, CO3, SO4, Cl, NO3 of Ca, Mg, Na, K,, Fe, etc)
Uses of water
Water from all the sources has the following main uses
- Firstly, for drinking and household purposes
- For boiler (steam generation, processing)
- Also for Industrial uses
- Equally, for irrigation purposes.
| Characteristic | Surface water | Groundwater |
| Turbidity | High | Low |
| Dissolved minerals | Low | Low-moderate |
| Biological impurities | High | low |
| Chemical impurities | High | low |
Potable water, for drinking and household purposes
In general, the water which is good for drinking is called potable water. This water has no suspended impurity or carries to the purification of water. Regardless, this water should be colourless, odourless and free of bacteria. It should also have some present taste (usually sweet). Pure water is not suitable for drinking, some minerals should be there but not too much to injurious to health. The world health organization (WHO) has given the following physical standards for drinking water also which are safe limits.
| Turbidity | 5ppm |
| Colour | 15 Hazen units |
| Odour | 3 threshold number |
| Taste | Pleasant (sweat usually) |
| T.D.S | 500 ppm |
| Chlorine | 250 ppm |
| Sulpher oxides | 250 ppm |
| Furious | 0.3 ppm |
| Si | 3 ppm |
| Mn | 0.05 ppm |
| Total hardness | 25 ppm |
Also, the above discussed are the safe limits of drinking water and have been accepted universally.
So, if water has some impurities and is not suitable for drinking it should be purified.
A benchmark in consumption resources and energy
| Process | Electrical energy KWH/Kg textile substrate | Thermal energy MJ/Kg textile substrate | Water consumption L/Kg textile substrate |
| Wool scouring | 0.3 | 3.5 | 2-6 |
| Yarn finishing | – | – | 70-120 |
| Yarn dyeing | 0.8-1.1 | 13-16 | 15-30(dyeing) 30-50 (rinsing) |
| Dyeing loose fibres | 0.1-0.4 | 4-14 | 30-50 (dyeing) 4-20 (rinsing) |
| Finishing knitted fabrics | 1-6 | 10-60 | 70-120 |
| Finishing woven fabrics | 0.5-1.5 | 30-70 | 50-100 |
| Finishing dyed knitted fabrics | – | <200 |
What is ppm of water?
It has the abbreviation of parts per million. It is a unit of contamination and chemicals and salts in a volume of water. Also, It is described as milligrams per litre (mg/l).
What is the TDS of water?
It is an abbreviation of a total dissolved solid. It is the total concentration of dissolved substances in drinking water. TDS are inorganic salts and also a small amount of organic matter. Also, these dissolved solids are sodium salt magnesium salts etc.